Abstract
Background:
β-thalassemia (BT) is a hereditary hemolytic anemia. The imbalance between α- and β-globin chain synthesis results in ineffective erythropoiesis, severe microcytic hypochromic anemia and iron overload. Although regular transfusions and iron-chelation therapy markedly improve the survival and quality of life of BT patients, they have also led to the emergence of previously unrecognized complications. Patients with thalassemia major often present with endocrine abnormalities due to dysfunction of the hypothalamic-pituitary axis (Poggi, 2016), and are frequently underweight with abnormally low body-fat percentage (Fung, 2010). Adipose tissue stores lipids, but it also has an endocrine function through the production of leptin, an adipocyte-derived hormone that regulates food intake, metabolic and endocrine functions by activating its receptor in the central nervous system. Leptin plays a regulatory role in immunity and inflammation, and also seems to act synergistically with erythropoietin on mammalian hematopoiesis (Bennett, 1996). For example, a stimulatory effect of leptin on erythropoiesis among patients with end-stage renal disease was observed (Axelsson 2005). Previous studies on leptin in BT patients have shown lower levels than in healthy controls, and a negative correlation with the level of soluble transferrin receptor in transfused patients (Dedoussis, 2002). The reduced leptin levels were explained by a possible toxic effect of iron on adipocytes (Chaliasos, 2010).
Study aims:
In this study, we investigated the correlation between leptin level and anthropometric parameters in BT patients compared to heathy controls. We also explored the relationship between leptin and hematological and erythropoietic parameters, as well as iron-overload status in BT patients.
Patients and methods:
Transfusion-dependent BT patients (n = 33; 16 females, 17 males, mean age 23.5 ± 8.6 [range 8-41] years), treated at the Pediatric Hematology Unit of Emek Medical Center, and 11 healthy controls (6 females, 5 males, mean age 26.4 ± 10.4 [range 8-39] years) were studied. Patients were treated by regular blood transfusions and iron-chelation therapy. Anthropometric assessments included height, weight, fat percentage and BMI calculation. Blood samples were obtained at fasting and before blood transfusion. Serum leptin, complete blood count, hemoglobin and reticulocyte counts and serum erythropoietin were analyzed. These studies were performed twice, 3 months apart, and the mean values were utilized for the statistical analysis. Serum leptin levels were analyzed by radioimmunoassay, and leptin-normalized values were calculated (ng/dL leptin per % body fat).
Results:
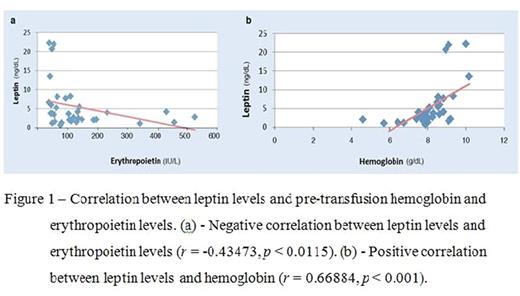
BT patients were found to have lower leptin levels than healthy controls (5.4 ± 5.9 vs. 13 ± 10.1 ng/dL; p= 0.0006). Leptin levels were higher in females than in males (mean leptin 7.2 ± 6.5 vs. 3.7 ± 4.7 ng/dL; p= 0.01), and increased with age (r= 0.4635, p= 0.0066). A negative correlation was found between leptin and erythropoietin (r= -0.43473, p= 0.0115), and a positive correlation between leptin and hemoglobin, as well as the mean pre-transfusion hemoglobin levels in the previous 5 years (r= 0.66884, p < 0.001; r= 0.40261, p= 0.0202, respectively). No correlation was observed between leptin levels and anthropometric parameters (weight, height, BMI), iron-overload parameters or reticulocyte count. Fasting and normalized leptin showed similar patterns.
Conclusions:
This study confirms that BT patients are unable to maintain adequate leptin production, suggesting that adipose tissue dysfunction may be related to the chronic anemia. Our results correlate well with previous studies of lower leptin levels in BT patients. The positive correlation of leptin level with hemoglobin, together with the inverse correlation with erythropoietin provide further evidence of the effect of leptin on erythropoiesis. Additional studies are needed to examine the intricate interplay between adipose tissue, leptin and erythropoiesis in the environment of chronic anemia and iron overload present in BT patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal